Adjunct Professor Denzil Ferreira, at the Centre for Ubiquitous Computing has been awarded funding from the Academy of Finland’s ICT 2023 programme, for project STOP: Sentient Tracking Of Parkinson’s.
About STOP:
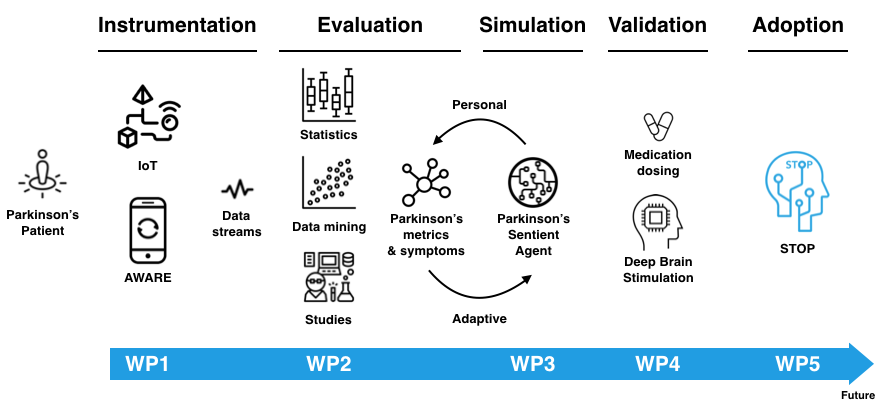
Effective monitoring of patients is technically challenging with wearables due to: 1) battery life is limited to a few hours when sampling and processing data continuously; 2) it is not feasible to process large amounts of sensor data on a wearable (e.g., a smartwatch). Instead of wearables, we leverage widely available technology such as smartphones, and Internet-of-Things (IoT) sensors, and augment their sensing capabilities with user-centric machine learning to create an infrastructure that links medication alerts to movement phenomena in real-time, to improve medication dosing for Parkinson’s patients and to unobtrusively monitor the progression of Parkinson’s Disease (PD).
STOP makes possible PD real-time symptom tracking, empowers DBS (Deep Brain Stimulation) patients with intelligent micro-adjustments of neurostimulators’ electrical stimulation, and provides medication dosing awareness and reminders for improving medication compliance. STOP provides valuable PD information for clinicians (e.g., a personal understanding of medication dosing lasting effects, the impact of PD symptoms in daily tasks, potential changes in mobility and social behaviour), thus potentially improving the efficiency of PD health services. STOP can minimize travel: clinically relevant data can be gathered and delivered remotely to clinicians and health researchers, allowing them to intervene, study and better understand PD; monitor PD unobtrusively over clinically relevant time periods: sensor data is gathered autonomously over long periods of time to better understand the manifestation of PD symptoms during day-to-day activities and inform future interventions and guidelines; provide clinically predictive modelling of medication need: intelligent personal modelling of users will allow for tailored PD support, particularly when PD symptoms are troublesome and require medication and DBS intervention.
Following AWARE’s plugin-based architecture, STOP will be a scalable, battery-conscious, cost-effective PD monitoring solution that uses commercially available smartphones and IoT sensors to collect and judiciously send data to a cloud infrastructure to improve PD patients’ quality of life. STOP will transform the care of PD by enabling an ecosystem of support involving patients, families, caregivers, clinicians and health researchers, working together. By monitoring the progression of PD, clinicians can better understand the effects of the medication, users’ lifestyle and individual Parkinson’s progression. Effective monitoring, analysis of movements and predictive modelling can be used by clinicians to send real-time medication alerts, improve medication adherence, and monitor PD patients’ quality of life from afar, also applicable for other neurological disorders, such as dementia, Alzheimer’s and Huntington’s.
Project STOP has the duration of two years, starting January 2018. The project is the fruit of the collaboration with Haltian Oy, Head Instruments Oy, University of Oulu Medical Faculty (Dr. Jani Katisko), Carnegie Mellon University (Prof. Anind Dey, Julian Ramos), The University of Manchester (Prof. Simon Harper, Julio Vega) and is lead by Adj. Prof. Denzil Ferreira from the Center for Ubiquitous Computing at the University of Oulu.
WP1: Develop methods and tools that measure PD symptoms unobtrusively
BSc. Valerii Kan, a MSc Thesis Research Assistant working in this project, has created our first tool that leverages gamification as a method and Android smartphones as a tool to track and log medication adherence for Parkinson’s patients. The application acts as a proxy to assess medication lasting effects throughout the day and self-reported symptoms. The work is now being evaluated by non-PD participants for establishing the baseline of non-afflicted subjects.